Isothiocyanate couples well with a reactive amino group in a protein and peptide chain under mild conditions. Isothiocyanatofluorescein is widely used in labeling different biomolecules such as immunoglobulins, lectins, proteins, peptides, nucleic acids, oligo-and polysaccharides. The derivatives of FITC are also extensively utilized in biological analyses [1].
Fluorescein isothiocyanate (FITC) is the most widely used fluorescent probe for the preparation of conjugates with biological molecules. This xanthene dye is particularly useful for several reasons: conjugates are easily prepared because of the water solubility of FITC; it is brightly fluorescent because of its reasonably large extinction coefficients and high quantum yields after conjugation; and it has low nonspecific binding with most biological tissues. The preparation of fluorescein conjugates from the closely related (4,6-dichlorotriazinyl) aminofluorescein (DTAF) has been recommended. Fluorescein is maximally excited by blue light and emits primarily green to yellow fluorescence.
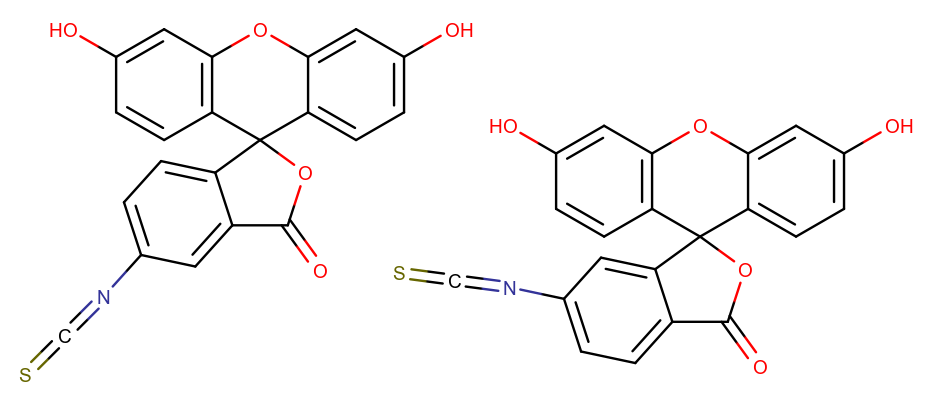
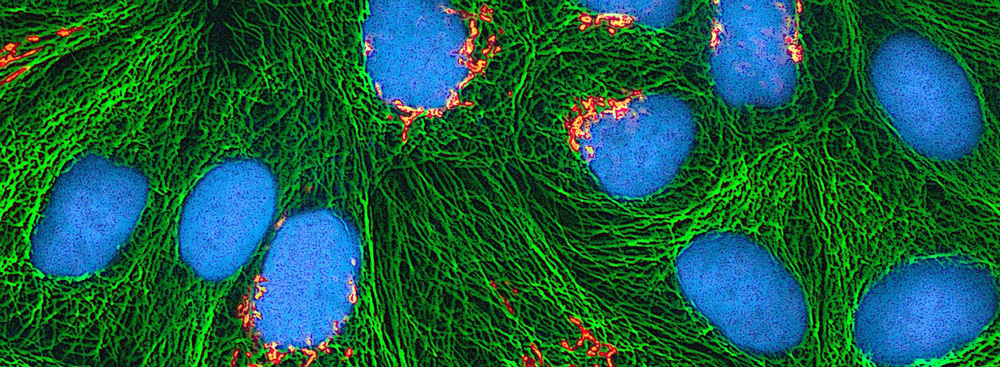
Fluorescein is a fluorescent tracer with wide applications in laboratory research and medical practice (fluorescein angiography [2] and fluorescein-guided surgery [3] ). In water, its maximum absorption/emission is 494/518 nm, corresponding to the color blue and green. The blue color spectrum is 450-495 nm, and the green spectrum is 495-570 nm. Fluorescein isothiocyanate (FITC) is the derivative of fluorescein with an isothiocyanate reactive group
Our fluoresceines
References
[1] DOI: 10.2174/157019309787316111
[2] DOI: 10.1016/j.ophtha.2017.08.005
[3] DOI: 10.1158/1078-0432.CCR-17-1184

