The six-spined spruce bark beetle, Pityogenes chalcographus (in Germany called Kupferstecher), is a serious pest in Europe of Norway spruce (Picea abies), especially the younger trees. Both sexes of these tiny beetles (2 mm long) aggregate on host trees in response to a pheromone. [1] Chalcogran, 2-ethyl-1,6-dioxaspiro[4.4]nonane, is a principal component of the aggregation pheromone of the bark beetle Pityogenes chalcographus. [3]
All stereoisomers of chalcogran
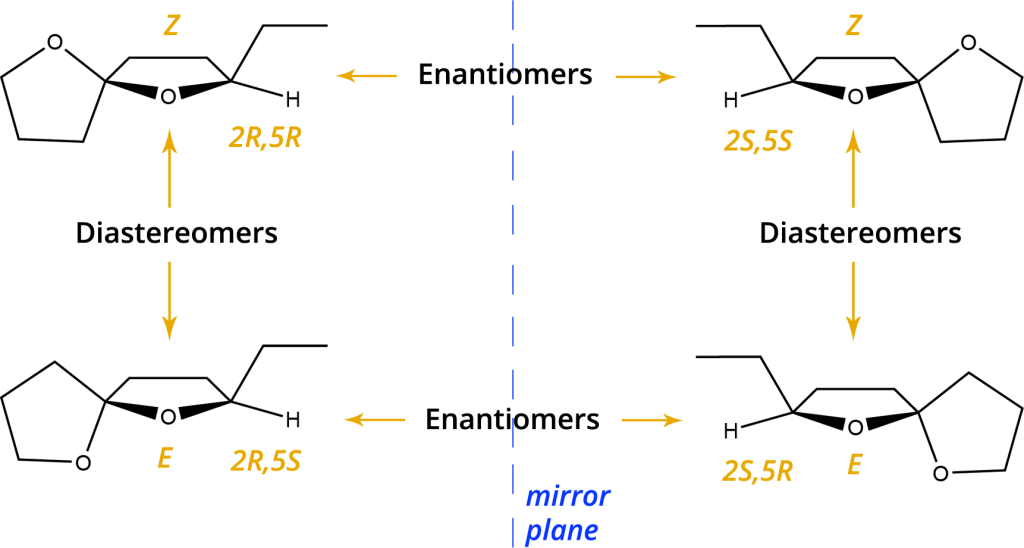
The molecule comprises two stereogenic centres resulting in four possible stereoisomers which differ in their biological activity, the natural chalcogran being a mixture of only two diastereomers, 2S,5S and 2S,5R. [3] The two natural enantiomers found in male Pityogenes chalcographus are to the right of the mirror plane, with the behaviorally active one in the lower right. The diastereometric (2S,5R) and (2S,5S) mixture has been isolated from the beetle. [2]
Spiroacetals in Insects
Spiroacetals are relatively widespread among insects. In total, 30 different structures (not including stereoisomers) have been found in beetles (Scolytidae, Cerambycidae and Staphylinidae), in ants, bees (solitary and social species) and wasps, in bugs and many fruit flies, Bactrocera species. In some insects, behavior mediating properties are associated with spiroacetals: they are intraspecifically used as pheromones and interspecifically as kairomones and possibly as allomones, however, in most cases their biological significance is still unknown.
Substituted spiroacetals may occur as (E)- and (Z)- diastereomers. When the substituent and the spiro-oxygen of the alternate ring are on the same side of the reference plane, the isomer is called (Z)-, if not, it is the (E)-isomer. The reference plane is the substituted ring. The first spiroacetal identified in insects was 2-ethyl-1,6- dioxaspiro[4.4]nonane, chalcogran, a component of the male produced aggregation pheromone of the European spruce bark beetle, Pityogenes chalcographus. Chalcogran was also identified in the closely related bark beetle, Pityogenes quadridens. Racemic chalcogran (Cas number: 38401-84-2) proved to be strongly attractive to a predator, the Ostomid beetle, Nemosoma elongatum. A racemic formulation is used commercially in integrated forest pest management. Last but not least, chalcogran was found to be a component of the odor bouquet of flowers of Dracaena fragrans and several orchid species. A few homologues of chalcogran have been identified from solitary bees, Andrena species, however, their biological function is unknown. [4]
References
[1] Byers, J.A., Högberg, HE., Unelius, C.R. et al. J Chem Ecol (1989) 15: 685
[2] Acta Chemica Scandinavica 41b(9):694-697 · October 1987
[3] Chem. Pap. 68, 745–750 (2014)
[4] Current Organic Chemistry, Volume 5 , Issue 2 , 2001




